Support
Support
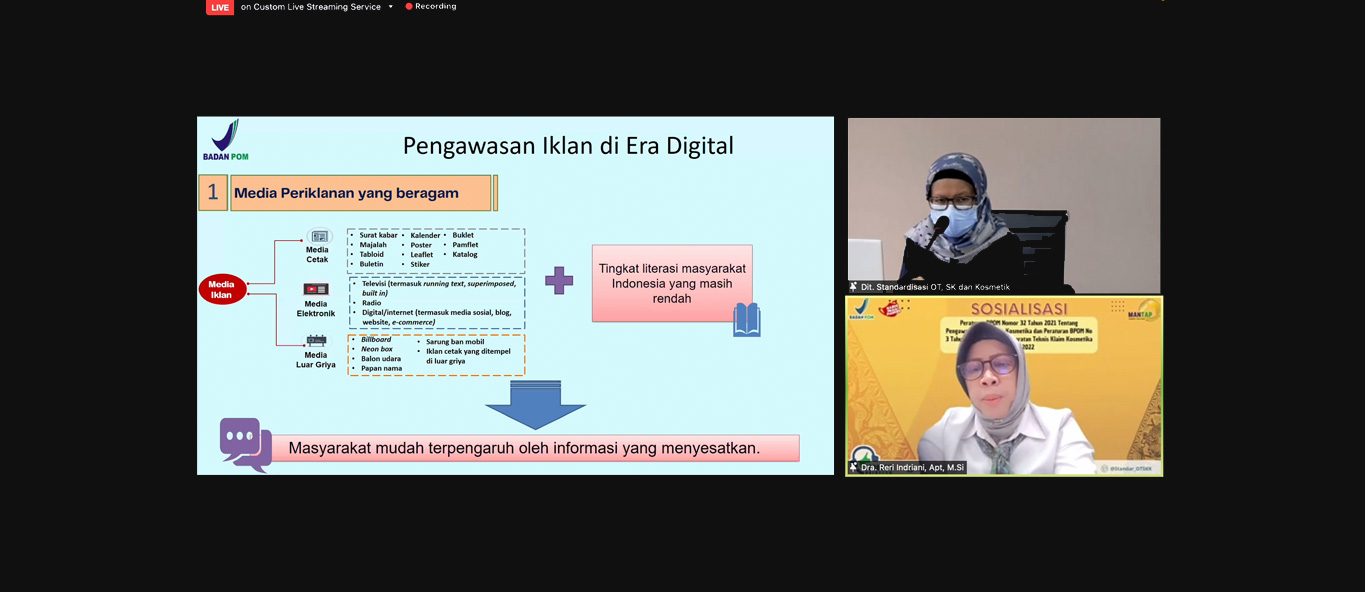
Socialization of BPOM Regulation No. 32 of 2021 on Supervision of Cosmetic Advertising and BPOM Regulation No. 3 of 2022 on Technical Requirements of Cosmetic Claims
Last Tuesday, March 1, the Food and Drug Supervisory Agency of the Republic of Indonesia held a Socialization of BPOM Regulation No. 32 of 2021 on Supervision of Cosmetic Advertising and BPOM Regulation No. 3 of 2022 on Technical Requirements of Cosmetic Claims. This regulation is designed to ensure the safety, usefulness and quality of cosmetics for the public, as well as prevent the presence of cosmetic products that do not meet technical requirements and make misleading or exaggerated claims on the market.
This regulation regulates the requirements, ordinances, advertising actors, warning information that must be included in the advertisement of certain cosmetic products, to administrative sanctions that will be imposed if the business actor is caught violating the applicable advertising rules and cosmetic claims. Since it was officially promulgated in December 2021, business actors are obliged to adjust to the provisions in this new regulation no later than 6 months from the date the regulation was promulgated, which is June 13, 2022. IdEA member companies that manufacture and/or advertise cosmetic products are urged to immediately comply with the new regulations to avoid sanctions and reprimands.
translation-not-found[latest_article_idea]
01



